Unmet medical need
Developing meaningful therapies for indications in virology & immunology
Developing meaningful therapies for indications in virology & immunology
Creating higher values for patients, health systems & stakeholders
Marinomed has partnerships in > 40 countries
Marinomed's vision is to transform the lives of people suffering from diseases with limited or no treatment options in two key therapeutic areas: virology and immunology.
Therefore, it is our mission to provide patients and physicians with powerful technologies that significantly improve patients' quality of life. Our proprietary and validated platform, Marinosolv, provides the basis for novel medicines to treat indications with unmet medical needs.
With our passion for scientific progress and our expertise in respiratory, infectious, immune, and eye diseases, we strive to create sustainable value for patients, health care systems, the Company, and our stakeholders.

We pursue a strategy based on a mix of commercializing our late-stage assets, developing our own pipeline products, and offering formulation development services for our Solv4U customers. Our strategic priorities are as follows:
Strengthening our OTC business through new partnerships and by supporting existing partners in market access with current and future products in both established and new markets;
Expanding business development activities focused on assets that are sufficiently advanced to initiate partnering discussions. This includes our late-stage pipeline projects such as Budesolv (MAM-1004-1) and Tacrosolv (MAM-1003-1), as well as newly developed OTC products in the immunology space;
Long-term adherence to our mission by inventing, developing, and selecting promising pipeline programs for indications with high unmet medical needs, funded through the Company’s cash flows.

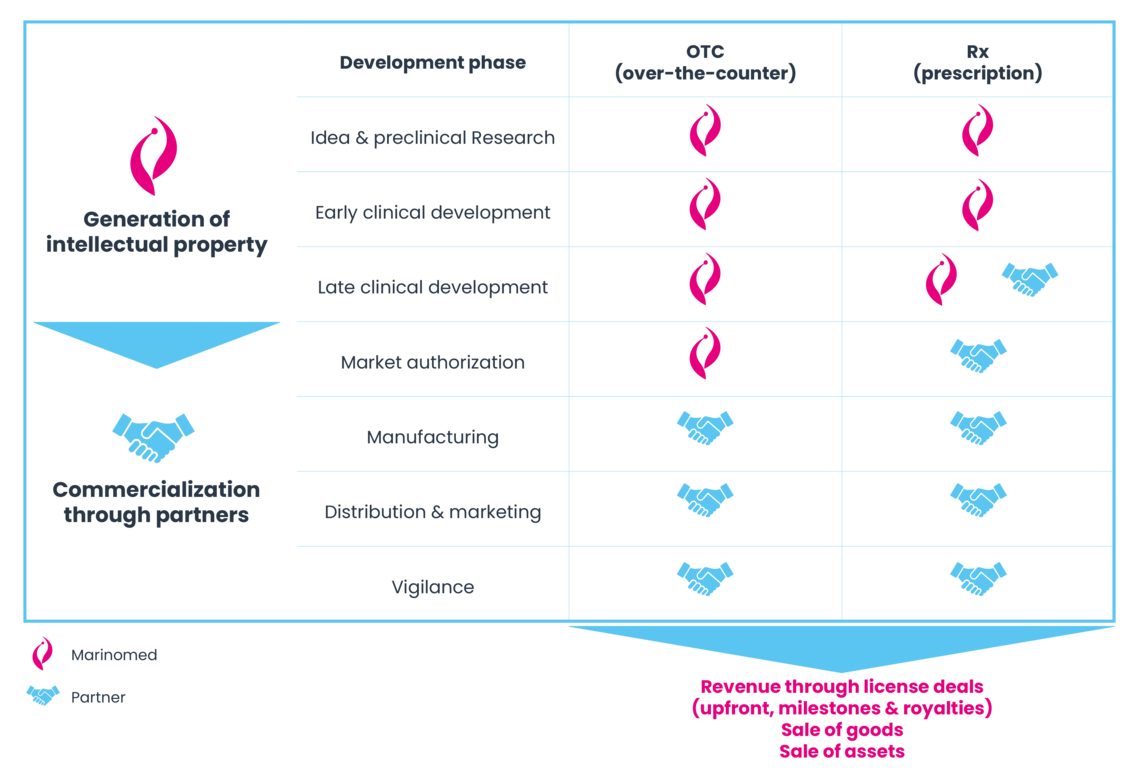
Marinomed develops pharmaceutical products and medical devices in the therapeutic areas of virology and immunology. The Company’s core competencies lie in preclinical and early-stage research and development, with a strong focus on generating intellectual property. In the field of pharmaceutical products—primarily based on its proprietary Marinosolv® technology—Marinomed collaborates with industry partners by granting licenses during the clinical development phase.
The Company develops non-prescription (OTC) medical devices up to market approval. These products are then manufactured by contract manufacturing organizations and outlicensed to partners who handle global marketing and distribution. Marinomed’s commercial partners are mainly well-established pharmaceutical companies with territorial licensing agreements. With a lean supply chain structure, the Company currently manages 20 commercialization partnerships across more than 40 countries in the OTC segment (as of 31 Dec 2023).
For the development of prescription (Rx) pharmaceuticals, Marinomed aims to secure strategic partners during or after Phase II clinical trials. In these highly regulated and specialized markets, collaboration with financially strong partners that bring therapeutic expertise and regulatory experience is critical to success.
The Company’s objective in the Rx segment is to establish traditional pharmaceutical licensing agreements. Securing Luoxin Pharmaceutical as a partner marked an important first step. These partnerships typically include upfront, milestone, and royalty payments, while commercialization responsibilities—from manufacturing to distribution—remain with the licensee. This allows Marinomed to focus on its core strength: delivering high-value research and development.
